Dense bracken groves suppress ground vegetation, hinder biodiversity, and release toxins, posing a challenge for nature managers. Traditional approaches to control bracken growth have involved manual cutting and the use of the herbicide “Asulam.” However, recent trends have shifted away from artificial interventions and toward the restoration of natural processes. This raises the question: is there a rewilding-based solution to address the issues associated with bracken?
This article is written by Søren Thomsen, co-founder and writer at The Extinctions and National Contact for Denmark for the European Young Rewilders. He has an MSc in Nature Management from Copenhagen University and runs a small agro-rewilding-tourism project called Sjælegård along with his family.
With the ongoing global biodiversity crisis and significant land utilization for food production and resource extraction, the restoration of remaining natural areas has become imperative. Throughout Europe, many “natural” systems have experienced severe degradation, leading to an imbalance caused by the absence of crucial natural processes. A striking example of this ecological disequilibrium is the overdominance of common bracken. Dense bracken groves suppress ground vegetation, hinder biodiversity, and release toxins, posing a challenge for nature managers. Traditional approaches to control bracken growth have involved manual cutting and the use of the herbicide “Asulam.” However, recent trends have shifted away from artificial interventions and toward the restoration of natural processes. This raises the question: is there a rewilding-based solution to address the issues associated with bracken?
Understanding the Ecology of the Common Bracken
Before discussing how to tackle the common bracken (Pteridium aquilinum) it is essential to delve into its intriguing ecological dynamics. Renowned for its exceptional adaptability, the common bracken thrives in diverse environments, spanning from the Arctic circle to the tropics, and from high-altitude alpine regions to lowland habitats at sea level (9). The sheer geographical extent of common bracken is tremendous, encompassing parts of Europe, Asia, Africa, Oceania, and the Americas (9). Its capacity to endure various soil conditions, moisture levels, light intensities, and temperatures positions it as an extraordinarily resilient species in the plant kingdom.
The common bracken’s resilience stems from its distinctive adaptations, which enable it to dominate the areas it occupies. While the fronds of the bracken constitute only a fraction of its biomass, up to 85% of each plant’s mass is stored in underground rhizomes (9). These rhizomes not only serve as storage organs but also facilitate nitrogen fixation, enriching the soil and supporting the bracken’s survival in low-nutrient environments (10).
Moreover, the common bracken exhibits remarkable resistance to various disturbances, including trampling, storms, and wildfires, thanks to its underground rhizomes and their ability to sustain the plant even when above-ground portions are damaged (7, 8). Furthermore, bracken’s dense clusters of fronds can shade out undergrowth, obstructing light and hindering the establishment of competing plant species (8, 9). In colder regions, the bracken wilts and dies in autumn, leaving behind litter that persists year-round, further impeding light penetration (8).
The bracken’s toxic compounds, thiaminase and ptaquiloside, play a pivotal role in its defence mechanism and ecological interactions. Thiaminase has the potential to break down thiamine in animals that ingest it, leading to thiamine deficiency, which can be fatal, particularly in animals consuming significant quantities of bracken (4, 9, 12, 21). Additionally, the compound ptaquiloside possesses highly carcinogenic properties and has been linked to the development of stomach and oesophageal cancer in animals and humans (4, 9, 12). The precise mechanisms by which these toxins affect organisms, and the environment are not yet fully understood, but their presence serves as a potent deterrent against grazing and may also impact bacterial, floral, and fungal communities (9).
In addition to these adaptations, the common bracken possesses dispersal mechanisms that contribute to its colonization and expansion. The growth of rhizomes allows new fronds to sprout adjacent to existing ones, facilitating slow but potent invasion through the nutrient network of pre-existing rhizomes. Additionally, spores enable the colonization of new areas, albeit with lesser vigour compared to rhizome expansion (9).
The extraordinary adaptability and resilience of the common bracken have enabled it to dominate natural ecosystems, leading to its often-cited categorization as a “pest” species.
Is Bracken *really* a problematic species?
Challenging the notion of common bracken as an inherently detrimental species is worth considering. While bracken’s adaptability allows it to occur widely, its dominance is limited to specific ecosystem types, such as light-open forests, heathlands, and certain grasslands (9). These ecosystems are however relatively rare and typically hold conservation value. Bracken also tends to invade abandoned agricultural and grazing lands, as well as areas affected by logging activities (9) and may hamper restoration efforts. There is speculation that bracken’s dominance might be temporary due to the accumulation of bracken litter in the soil profile. Over time, this litter may reach the depth at which rhizomes grow, creating an unfavourable growth medium for the ferns. However, this process is not yet fully understood and can take several decades (9).
Despite its dominance, bracken does not completely eliminate undergrowth species. Depending on the habitat, it often forms communities with another (or sometimes multiple) common species. In Northern Europe these may include brambles (Rubus spp.), wavy hair grass (Deschampsia flexuosa), or heath bedstraw (Galium saxatile) (9). In some woodland ecosystems, shade-tolerant undergrowth species can coexist within dense bracken groves (9). However, these plant communities within dense bracken groves typically exhibit low biodiversity (9).
It is important to distinguish between dense bracken groves and common bracken in general. As a native species in Europe, common bracken forms mutualistic relationships with various species, particularly moths and butterflies that rely on the ferns as food for their larvae (20). Consequently, common bracken can contribute to biodiversity when part of a more diverse flora but be detrimental in dense swathes.
From an anthropocentric perspective, bracken’s inherent toxicity poses problems. Consumption of bracken is part of the cuisine in certain regions, such as Japan and South America, where it has been linked to high rates of stomach and oesophageal cancer due to ptaquiloside (14, 16) Ptaquiloside can also enter the food chain through the milk and meat of livestock grazing in bracken-infested areas (19). Moreover, high concentrations of ptaquiloside has been found in groundwater beneath bracken groves, rendering such water sources unusable (3). These circumstances raise ethical concerns regarding animal welfare in free-range grazing. Although grazers generally avoid consuming bracken (Pers comms), they may be compelled to eat it under over-grazed conditions.
Therefore we can conclude that bracken isn’t problematic per se, but under optimal conditions it can become ubiquitous and damage both biodiversity and important ecosystem services. Having explored the ecological dynamics and potential impacts of common bracken, the focus must be shifted towards the management methods commonly employed to control its spread.

Fig 1. Common bracken invading a newly deforested patch at Sjælegård, a small agro-rewilding project in Bornholm, Denmark.
Conventional Management
Conventional management approaches for common bracken have primarily involved cutting the fern stems or using the herbicide Asulam. Cutting the bracken stems, typically done annually or biannually, has shown success in reducing bracken densities (8, 10, 11). A study from a heathland in East Anglia showed that cutting bracken twice a year for 18 years, reduced bracken densities to just 3% of their original stand. Cutting works by forcing the plant to divert energy towards regrowth and deprive it of opportunities for photosynthesis. However, this method is labour-intensive, time-consuming, and above all reversible, as bracken populations quickly recover once cutting is ceased. An additional concern is that cutting has been shown to increase the production of ptaquiloside in bracken, worsening health impacts (13)
On the other hand, the use of Asulam as an herbicide requires multiple applications for long-term effectiveness (17), but its usage has declined due to a ban imposed by the European Union to address concerns related to human and environmental health. What’s more, studies have also typically found it to be less effective in curbing bracken than manual cutting (6, 8).
One study investigated the long-term effects of both asulam and cutting on species richness and undergrowth cover. Whilst both treatments were successful in limiting decreases in biodiversity in the short term, there were no long-term gains (1). Thus, it can be concluded that conventional methods have severe limitations and drawbacks, highlighting the need to explore alternative nature-positive approaches. Rewilding may offer just such an option.

Fig 2. Manual cutting of bracken using a bush clearer. Sjælegård, Denmark.
Answers in Rewilding?
Is it possible that the overdominance of bracken in some ecosystems is a result of missing natural processes? Absolutely. Two processes in particular should be considered: trampling and rooting. These processes hold the key to formulating potential solutions to bracken domination based on ‘rewilding’ principles.
Bracken, with its highly rigid stem, is easily trampled down, often resulting in its death. While the bracken regrows in most cases, frequent disturbances can prevent its establishment and allow more stress tolerant species to establish themselves. However, the absence of large animals in the European landscape today has reduced the prominence of trampling. The largest animal, the wisent, weighing about half a ton, is still absent from most of Europe’s nature areas. While the effect of trampling by wild herbivores on dense bracken groves has not been extensively studied, trampling by domestic cattle has been used as a tool for bracken control by strategically placing salt licks. This method mainly results in gaps along game trails and highly frequented areas, allowing young trees to establish and potentially outcompete the bracken by depriving them of light (9, 22). Therefore, introducing large animals into areas with bracken may mitigate their dominance to some extent. However, careful management is necessary to avoid overpopulation and the risk of poisoning. In smaller projects, the use of salt licks can also be considered. It is worth contemplating whether the trampling from extinct megaherbivores in Europe limited bracken dominance. Could the movement of a herd of European forest elephants or the daily commute of hippopotamuses from water bodies to grazing sites have prevented bracken establishment? Unfortunately, without a comprehensive understanding of the habits and natural densities of the European megafauna, it is impossible to draw definitive conclusions. Moreover, this discussion is largely academic given the animals in question and Europe’s current political climate.

Fig 3. Use of salt lick in a bracken grove at Sjælegård, Denmark. The bracken is incapable of growing in the constantly trampled forest floor, however the clearing is limited to the immediate adjacency of the salt lick.
The second mechanism to consider is rooting, which refers to the behaviour of animals digging up the ground in search of roots for food. Many species, including horses (2), engage in rooting to varying degrees, but no species in Europe exhibits this behaviour as heavily as the wild boar. The rhizomes of bracken, which store nutrients below-ground, contribute to its resilience and tolerance of disturbances above-ground. However, the rhizomes are vulnerable and their exposure to the elements is detrimental, and the plant typically dies as a result of rooting. The rhizomes of bracken also contain thiaminase and ptaquiloside, making them inedible to most animals. Wild boar, however, have evolved a resistance to bracken toxicity and actively seek out the rhizomes during autumn and winter when other food sources are scarce (15). A case study conducted in an Inverness Forest, where seven wild boar were released into a 13.5ha enclosure with pervasive bracken stands, demonstrated significant reductions in both bracken frond density (43%) and frond length (31.5%) between 2009 and 2011 (18). While comparative studies between areas with and without wild boar are lacking, similar findings have been reported in fenced wild boar projects in Denmark. Therefore, an effective strategy against bracken dominance could involve the conservation and restoration of wild boar populations across the continent. Areas struggling with pervasive bracken groves could increase accessibility to wild boar through measures such as wildlife corridors or relocations.
Introducing wild boar is a contentious issue in several European countries where they are locally extirpated, including the United Kingdom, Ireland, and Denmark. In such cases, it may not be feasible for the majority of landowners to introduce wild boar. However, domestic pigs can serve as a suitable alternative. Pigs are equally capable of preying on bracken. A study conducted at Burnham Beeches in England recorded an 83.5% decrease in bracken frond density and a 42.9% decrease in frond length over a 2-year period after the introduction of pigs (21). Similar effects have been reported in woodland and heathland sites across the UK (21). When introducing pigs or wild boar, caution must be exercised as they are not invulnerable to bracken poisoning. While pigs have demonstrated resilience to thiamine deficiency when given a moderate dose with access to supplementary food containing thiamine, pig deaths due to bracken poisoning can result from thiamine deficiency in rare cases (4, 5). Therefore, it is crucial to ensure that fenced animals have sufficient access to alternative food sources, either natural or supplemented. However, information regarding the long-term effects of ptaquiloside on pigs is currently lacking.
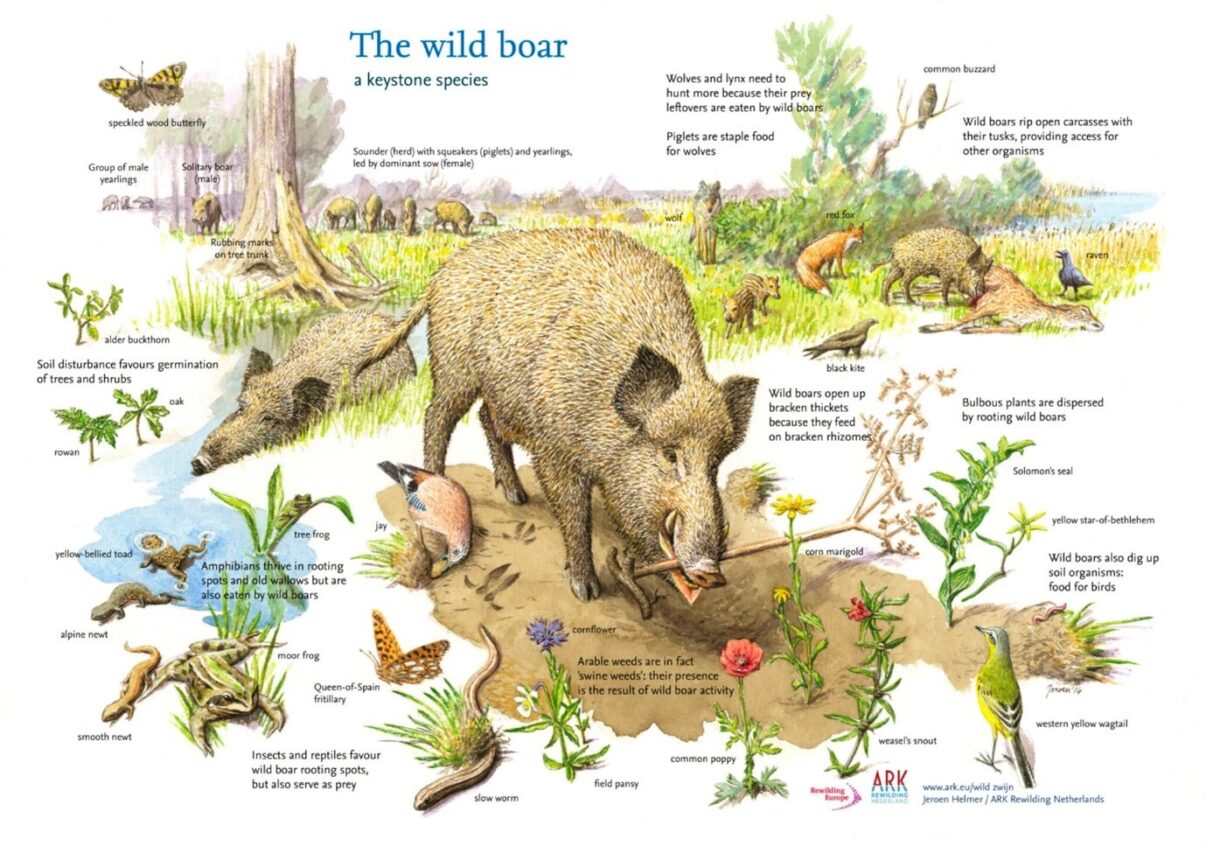
Fig 4. The Wild Boar is a European keystone species which performs a host of important functions on which other species rely. It is very effective at opening up bracken patches. Image credit to ARK Rewilding.
In conclusion, the issue of bracken dominance is worth addressing, but traditional management methods have shown limited long-term effectiveness and potential environmental risks. Rewilding presents a viable alternative by restoring trampling and rooting and creating favourable conditions for other plant species to thrive. The wild boar then is of particular interest in preventing bracken dominance and as a result would help ensure clean water, safe animal products and biodiversity. The disconnect of wild boar from bracken illustrates the need for a restoration of keystone species. Ecosystems in disequilibrium may be impoverished, expensive to maintain, or in the case of bracken – downright hazardous to human health.
References
- Akpinar, I., Alday, J. G., Cox, E., McAllister, H. A., Le Duc, M. G., Pakeman, R. J., Marrs, R. H. (2023). How long do bracken (Pteridium aquilinum (L.) Kuhn) control treatments maintain effectiveness? Ecological Engineering 186, 106842.
- Buttenschøn, R. M. (2007) Græsning of høslet i naturplejen. Miljø-ministeriet, Skov- og Naturstyrelsen, Center for Skov, Landskab og Planlægning, Københavns Universitet.
- Clauson-Kaas, F., Jensen, P. H., Jacobsen, O. S., Juhler, R. K., Hansen, H. C. B. (2015). The naturally occurring carcinogen ptaquiloside is present in groundwater below bracken vegetation. Environmental toxicology and chemistry 33(5), 1030-1034.
- Evans, L. A., Humphreys, D. J., Goulen, L., Thomas, A. J. (1963). Effects of bracken rhizomes on the pig. Journal of Comparative Pathology 73, 229-243.
- Harwood, D. G., Palmer, N. M. A., Wessels, M. E., Woodger, N. G. A. (2007) Suspected bracken poisoning in pigs. The veterinary record 160, 914-915.
- Le Duc, M. G., Pakeman, R. J., Putwain, P. D., Marrs, R. H. (2000) The Variable Responses of Bracken Fronds to Control Treatments in Great Britain. Annals of Botany 85, 17-29.
- Le Duc, M. G., Pakeman, R. J., Marrs, R. H. (2003) Changes in the rhizome system of bracken subjected to long-term experimental treatment. Journal of Applied Ecology 40, 508-522.
- Marrs, R. H., Le Duc, M. G., Mitchell, R. J., Goddard, D., Paterson, S., Pakeman, R. J. (2000) The Ecology of Bracken: Its Role in Succession and Implications for Control. Annals of Botany 85, 3-15.
- Marrs, R., Watt, A. S. (2006) Biological Flora of the British Isles: Pteridium aquilinum (L.) Kuhn. Journal of Ecology 94, 1272-1321.
- Milligan, G., Cox, E. S., Alday, J. G., Santana, V. M., Callister, H. A. M., Pakeman, R. J., Le Duc, M. G., Marrs, R. H. (2016). The effectiveness of old and new strategies for the long-term control of Pteridium aquilinum, an 8-year test. European Weed Research Society 56, 247-257.
- Paterson, S. (1996) Control and modelling of bracken (Pteridium aquilinum (L.) Kuhn in Great Britain. PhD Thesis, University of Liverpool, UK.
- Potter, D. M., Baird M. S. (2000) Carcinogenic effects of Ptaquiloside in bracken fern and related compounds. British Journal of Cancer 83(7), 914-920.
- Rasmussen, L. H., Donnelly, E., Strobel, B. W., Holm, P. E., Hansen, H. C. B. (2015) Land management of bracken needs to account for bracken carcinogens – a case study for Britain. Journal of Environmental Management 151, 258-266.
- Recouso, R. ., Stocco dos Santos, R. C., Freitas, R., Santos, R. C., de Freitas, O. B., Becak, W., Lindsey, C. J. (2003). Clastogenic effect of bracken fern (Pteridium aquilinum v. arachnoideum) diet in peripheral lymphocytes of human consumers: preliminary data. Veterinary and comparative Oncology 1 (1), 22-29.
- Sandom, C. J., Hughes, J., Macdonald, D. W. (2012) Rewilding the Scottish Highands: Do Wild Boar, Sus scrofa, Use a Suitable Foraging Strategy to be Effective Ecosystem Engineers. Restoration Ecology 21 (3), 336-343.
- Seawright, A. A., Smith, B. L. (1995). Bracken fern (Pteridium spp.) carcinogenicity and human health – a brief review. Natural Toxins 3 (1), 1-5.
- Stewart, G. B., Pullin, A. S., Tyler, C. (2007). The Effectiveness of Asulam for Bracken (Pteridium aquilinum) Control in the United Kingdom: A Meta-Analysis. Environmental Management 40, 747-760.
- Trees for Life (2011). Wild boar at Dundreggan. Unpublished.Wang, K. (2017).
- Virgilio, A., Sinisi, A., Russo, V., Gerardo, S., Santoro, A., Galeone, A., Taglialatela-Scafati, O., Roperto, F. (2015). Ptaquiloside, the Major Carcinogen of Bracken Fern, in the Pooled Raw Milk of Healthy Sheep and Goats: An Underestimated, Global Concern of Food Safety. Journal of Agricultural and Food Chemistry 63 (19), 4886-4892.
- Woodland Trust (2023). Bracken (Pteridium aquilinum). https://www.woodlandtrust.org.uk/trees-woods-and-wildlife/plants/ferns/bracken/
- Henney, J. (2012). An evaluation of the use of pigs as a method of bracken control. MSc Dissertation. Grazing Animals Project Heritage Grazing Traineeship.
- Marrs, R. H., Pakeman, R. J. (1995) Bracken invasion: lessons from the past and prospects for the future. Thompson, D. B. A., Hester, A. J., Usher, M. B. (1995). Heaths and moorlands: cultural landscape. Edinburgh: HMSO, 180-193.